Florence Computational Biology Group
(EXPLORE - P2022AB5TY, PRIN 2022 PNRR)
In questa pagina
Project details
Participating units:
Unit 1 - FONDI Marco - Università degli Studi di FIRENZE
Unit 2 – BRILLI Matteo- Università degli Studi di MILANO
Unit 3 – BURONI Silvia- Università degli Studi di PAVIA
Bacterial persistence (the capacity of some cells to survive antimicrobial treatments and resume growth afterwards despite being genotypically identical to sensitive ones) is currently a major concern in the fight against microbial infections. The factors driving the emergence of persisters are debated but it has been recently shown that there exists a relationship between ploidy (the number of genomic equivalents possessed by cells during their cell cycle) and the size of the persister sub-population. Ploidy represents a well-known intra-population heterogeneous trait that has been mainly explored in Escherichia coli and that has several drawbacks. These include the strongly unbalanced expression levels of genes located at the origin and terminus of fast growing active cells that possess more than one chromosome in specific moments of their cell cycles (e.g. Escherichia. coli) that, in turn, may lead to altered metabolic states. Also, it has been recently shown that poly-ploidy is related to the so-called phenotypic delay, i.e. the number of generations required for a cell to manifest a certain phenotypic property, such as an antibiotic resistance. Therefore, the frequency of persister cells can likely be reduced by first manipulating the ploidy of cells and then treating with the antibiotic. Given the economic and social impact of antibiotic persistence, we devise a thorough exploration of the issue in E. coli and extension to other pathogens with the aid of flow cytometry and mathematical modelling to discover general properties in the relationship of persistence with growth/metabolic state. Those models, once applied to in vivo data, would enable to quantify the expected degree of persistence allowing to devise personalized treatments. More in detail, we propose to study the characteristics of an important pathogen (Burkholderia cenocepacia) and model species (E. coli) in terms of ploidy in vitro to derive species-specific mathematical models able to provide estimates on cell cycle timing and metabolic heterogeneities in the population to be related to persistence. By developing a protocol for ploidy quantification in real-time by using the Fluorescence Activated Cell Sorter and successive -omics-based characterization of subpopulations, these models will provide a fast typing of the growth of the pathogens directly from human samples (e.g. blood or urine), before (to guide) or after (to monitor) antibiotic treatment. In this way, it will be possible to tune the antibiotic therapy on the bases of the growth rate and the metabolic state of the pathogens population in order to maximize its efficacy.
What we do in the project:
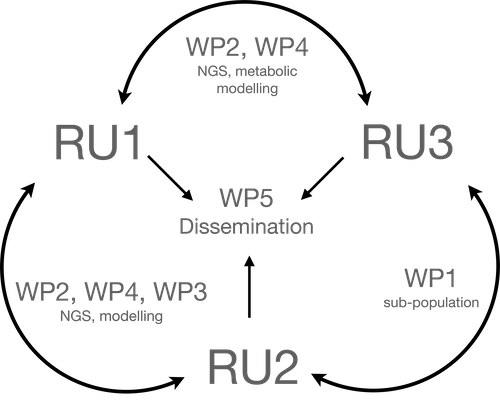
Current status of the project
As on May 2024, we have been mainly focusing on the model organism Escherichia coli. The following activities have been carried out:
1. Purchase of the reference strain E. coli and preparation of the glycerol stocks for downstream usage during the project. The setup of the persistence tests for the selected pathogens and antibiotics is ongoing, as well as the set up growth conditions for achieving different growth rates
2. Identification, design and order of probes for ploidy content estimation with flow cytometry. Design of fluorescent probes for the quantification of ori- and ter-proximal loci in the above species using flow cytometry has been carried out.
